Investigation of minerals behaviour in seawater for CO2 sequestration
The storage of CO2 is becoming increasingly important as its concentration increases in the atmosphere. Even if carbon dioxide emissions would stop tomorrow, we will still need to store carbon dioxide to keep the temperature increase well below 2°C and keep the commitment to the Paris Agreement.
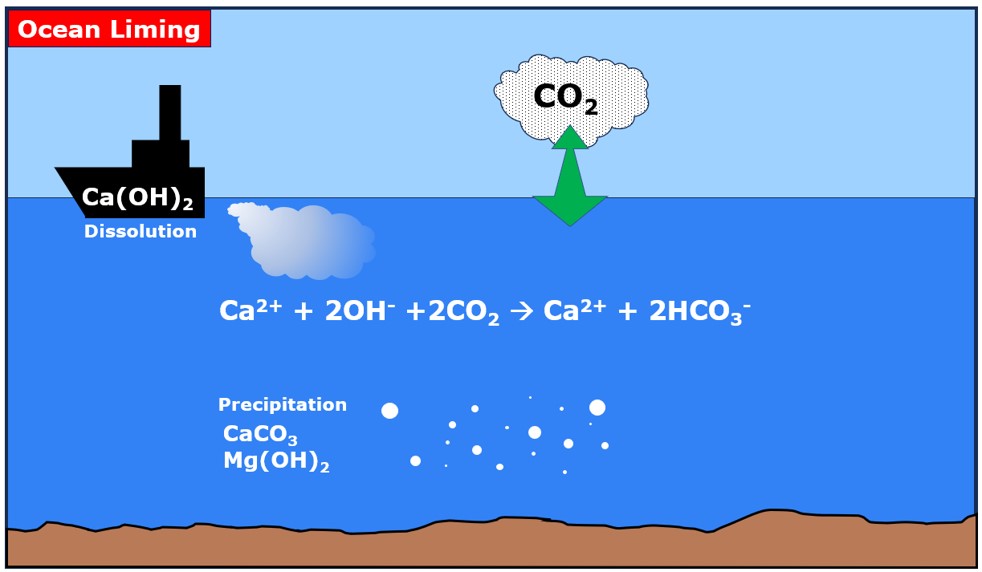
We study Ocean Alkalinity Enhancement (OAE), a branch of processes with great potential for storing CO2 in the oceans and decreasing the impacts of acidification by increasing their alkalinity.
Our projects focus on the use of calcium hydroxide with two application pathways:
• Directly in seawater to absorb carbon dioxide from the atmosphere.
• Dissolve in seawater captured CO2 from external sources (e.g. power plants), and then use Ca(OH)2 to buffer the seawater.
In both cases, the main adverse process is the precipitation of other alkaline minerals (aragonite, calcite and brucite). Our efforts focus on the analysis of the carbonate system in seawater to understand the dissolution and precipitation pathways of the minerals involved.
Laboratory and field campaigns
• From 06/2021, Ongoing – Small scale experiments in artificial and natural seawater, in our laboratory at the Polytechnic of Milan
• From 03/2024 to 06/2024 – Mesocosm experiment in collaboration with Limenet®
• From 06/2023 to 11/2023 – Industrial pilot plant experiment in collaboration with Limenet®
• In 06/2023 – Mesocosm experiment at HCMR, Heraklion, Greece
• In 10/2022 – Mesocosm experiment at ECIMAT, Vigo, Spain
Instruments
• Analitikjena Multi N/C 21005 for determination of inorganic carbon in solution
• HANNA instruments HI84531-02 titrator for determination of total alkalinity
• METTLER TOLEDO SevenExcellence pH-meter and conductimeter
• AERASGARD RFTM-LQ-PS-CO2 Modbus CO2 sensor
• Bruker D2-phaser powder diffractometer
• Rigaku-Synergy-S single crystal X-ray diffractometer
People involved
• Samira Jamali Alamooti (PhD student)
• Eleonora Kratter Thaler (Research Grant)
• Selene Varliero (PhD student)
• Prof. Piero Macchi
• Prof. Guido Raos
Partners
• University of Milano-Bicocca: Prof. Daniela Basso
• Politecnico di Milano, Department of Civil and Environmental Engineering: Prof. Arianna Azzellino
• University of Parma: Prof. Stefano Caserini
• Limenet®: Ing. Stefano Cappello, Ing. Giovanni Cappello, Dr. Francesco Pietro Campo, Federico Comazzi
Funding agencies and industries
• COnisma – Consorzio nazionale interuniversitario per le scienze del mare
• EuLA – European Lime Association
• Unicalce
• Fondazione Principe Alberto II di Monaco – OACIS project
Publications
• Varliero, S., Buono, A., Caserini, S., Raos, G., Macchi, P. Chemical Aspect of Ocean Liming for CO2 Removal: Dissolution Kinetics of Calcium Hydroxide in Seawater. ACS Engineering Au. 2024 in publication. link
• Caserini, S.; Cappello, G.; Righi, D.; Raos, G.; Campo, F.; De Marco, S.; Renforth, P.; Varliero, S.; Grosso, M. Buffered accelerated weathering of limestone for storing CO2: Chemical background. Int. J. Greenhouse Gas Control, 2021 112, 103517. link
Other references
• Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., … & Riebesell, U. Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches–consequences for durability of CO2 storage. Biogeosciences Discussions, 2022, 1-29. link
• Renforth, P., Henderson, G. Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics, 2017, 55, 636-674. link
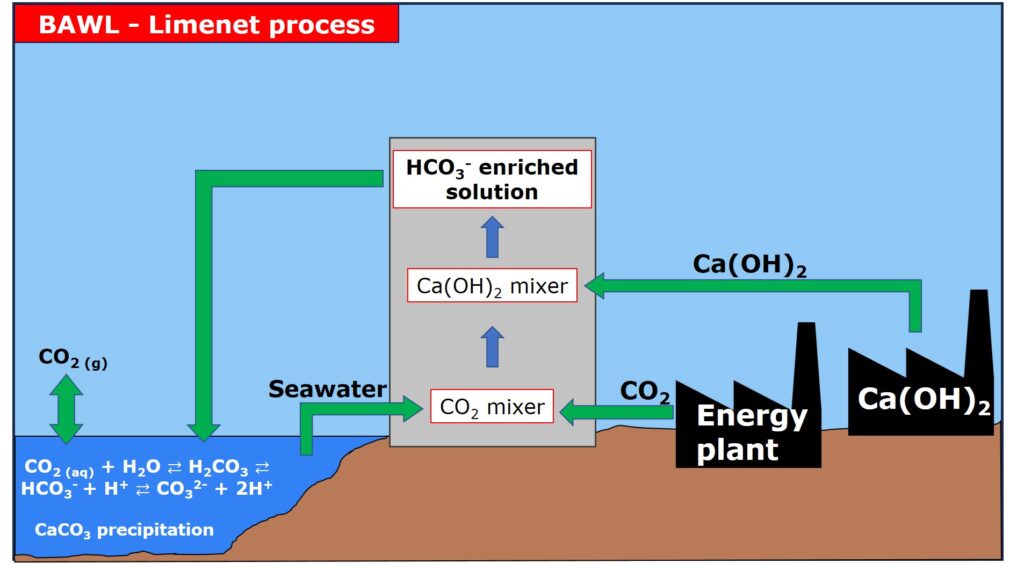
The BAWL Limenet process is the process invented and proposed by the company Limenet .




